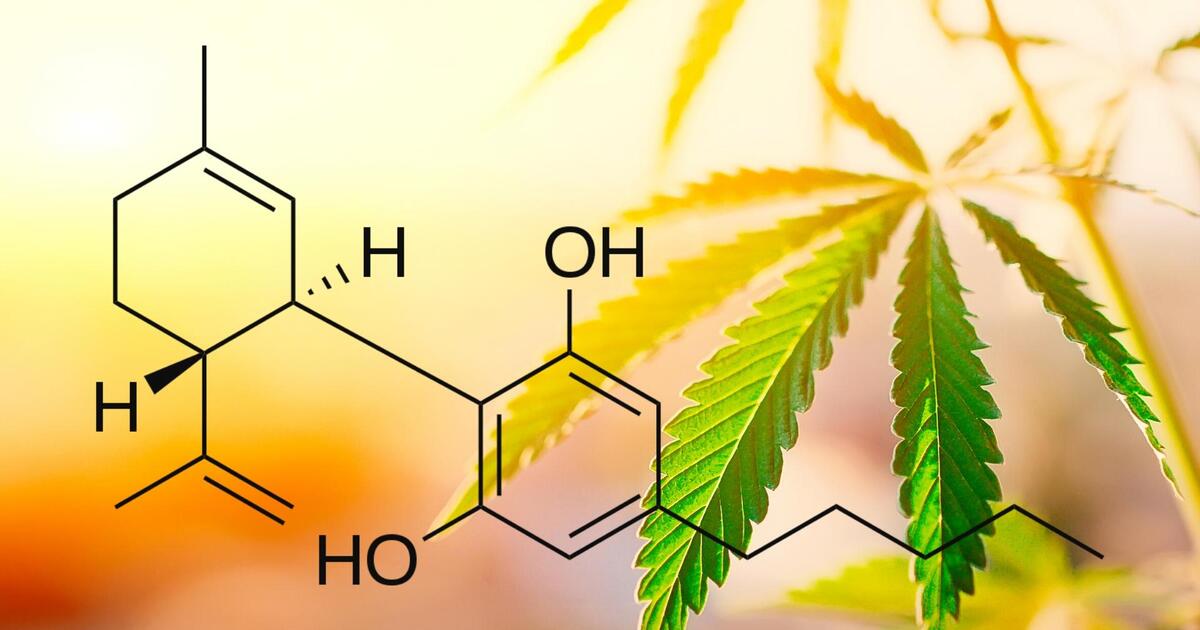
The global market for hemp, CBD, and other cannabinoids is taking off, and everyone involved — from farmers and processors, to distributors and retailers, to researchers and governments — needs experienced counsel to help them navigate the quickly evolving laws and regulations that govern it. Over the past decade, Vicente LLP has established itself as an international leader in hemp and cannabinoid law and policy. We have extensive experience with federal, state, and local hemp laws and regulatory programs, advising clients from every sector of the industry and assisting policymakers and regulators domestically and abroad to develop and implement sensible hemp policies.
Learn more: Vicente LLP State-by-State Hemp & Cannabinoid Compliance Guide
Our attorneys use this experience and institutional knowledge, as well as a cross-disciplinary approach that spans several of our practice areas, to provide clients with the skills and services they need to position themselves for success. Our Economics & Research department can analyze hemp and cannabinoid market dynamics to ensure our clients are making data-driven business and policy decisions, and our Corporate and Intellectual Property attorneys help get deals done and protect our clients' brands, innovations, and trade secrets. We also maintain collaborative relationships with international counsel in Canada, Mexico, and countries within the European Union and South America, allowing our clients to capitalize on opportunities not just throughout the U.S., but around the world.
To ensure our clients receive best-in-class guidance when it comes to navigating the intersection of hemp law with food and beverage law, consumer protection, and related issues, Vicente LLP has developed strategic relationships with firms that focus on U.S. Food and Drug Administration (FDA), Federal Trade Commission (FTC), and agricultural science matters. We are particularly excited to have formed an alliance with Kleinfeld, Kaplan & Becker LLP, a Washington, D.C.-based firm that concentrates on the regulation of products subject to the jurisdiction of the FDA — food, drugs and biologics, cosmetics, medical devices, dietary supplements, controlled substances, and tobacco products — as well as advertising law and other regulatory law governing consumer products and services. See the "Services" tab below for more details.