What Can We Learn from the FDA's Warning Letters for Hemp-Derived Products?
By Shawn Hauser, Angela George, Genevieve Meehan
Nov 24, 2025
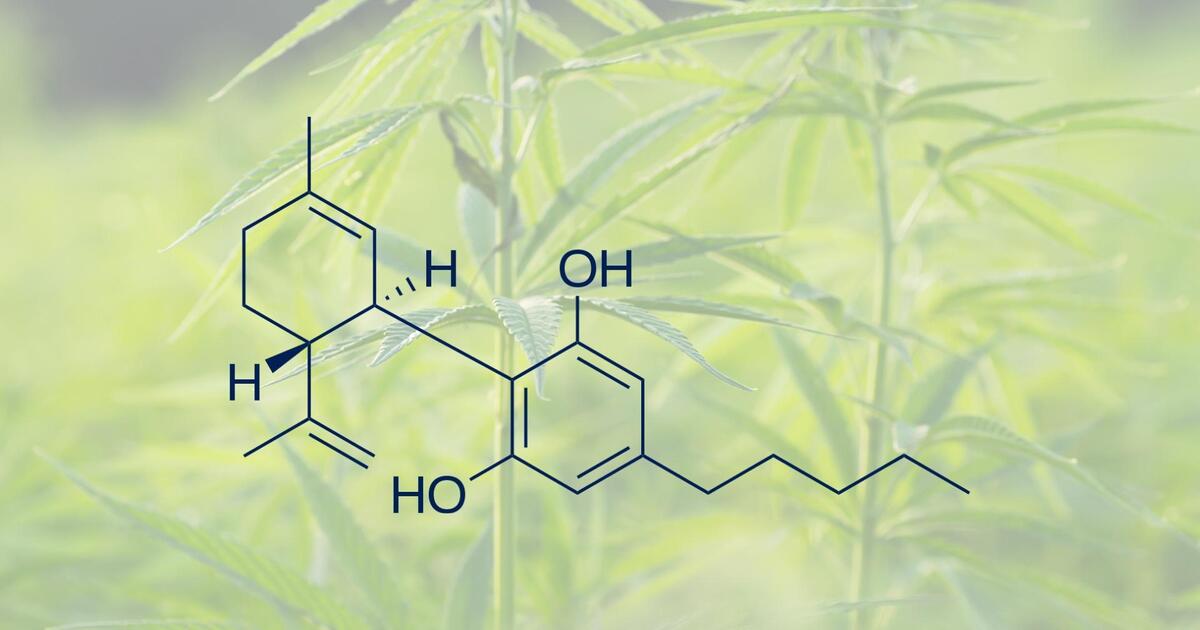
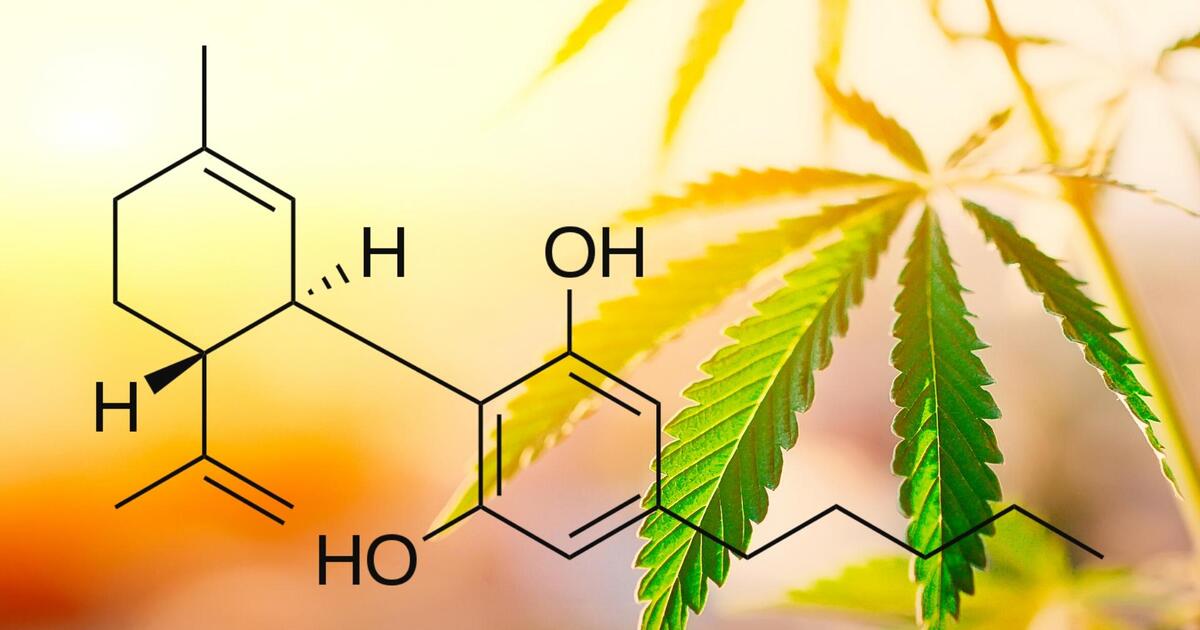
The FDA maintains that hemp-derived cannabinoids are not lawful food additives and has intensified enforcement against products making unapproved therapeutic claims, using unsafe or high-risk formulations, containing delta-8 in food, appealing to children through “copycat” packaging, or targeting pets and livestock. Recent warning letters reflect a clear shift toward stricter oversight across the hemp and cannabinoid sector. These measures highlight the rapidly evolving regulatory landscape and the need for companies to understand key enforcement themes from 2024–2025 and maintain strict compliance with federal and state law.